Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Diabetes Mellitus and Periodontitis: Risks and Challenges
*Corresponding author: Christopher H Turner MSc, BDS, MDS, FDSRCS, Retired Specialist in Restorative Dentistry, Bath, UK.
Received: October 27, 2022; Published: November 01, 2022
DOI: 10.34297/AJBSR.2022.17.002348
Abstract
Diabetics are at a 3 to 4 times higher risk of developing periodontal disease than non-diabetics. For diabetics who smoke the risk is 10 times. Many doctors are not aware of this. Diabetes mellitus and periodontitis are biologically linked, the one affecting the other and vice versa although the mechanism is not fully understood. Periodontal disease adversely affects glycaemic control. However, this control improves when periodontitis is successfully treated. Doctors should consider periodontitis for those patients with high HbA1c levels. Conversely, dentists should consider diabetes or prediabetes for patients with refractory periodontal disease. The two professions need to share results. This paper proposes a simple system of scoring for each disease using a traffic light, red, amber and green method. Pending a paradigm shift in inter-professional working, diabetics themselves should take charge of their results and share them with their respective advisors. Both doctors and dentists and their teams need to understand the significance of each other’s results and how they could impact on patient care. This is an educational challenge for the future for them and their patients. People living with diabetes who do not attend for dental care should be advised by their doctors about the potential benefits of dental screening.
Keywords: Diabetes; Periodontitis; Defining Risks; Records; Education
Introduction
The relationship between diabetes mellitus (DM) and periodontal disease (PD) is well established as a two-way process with one disease affecting the other and vice versa for reasons that are not fully understood. The diseases are somehow biologically linked [1]. Diabetics have a 3 to 4 times greater risk of developing PD than non-diabetics rising to 10 times for those who smoke [2].
While the pathophysiological mechanism of the relationship between the two diseases is still under investigation, there is a common pathogenesis involving an enhanced inflammatory response at both local and systemic levels caused by the chronic effects of hyperglycaemia and the formation of advanced glycation products that promote this inflammatory response [1]. Levels of cytokines [3], tissue necrosis factor [4] and C-reactive protein [5] are raised in both diseases. Toxic products from Gram negative bacteria initiate tissue breakdown and osteoclastic bone resorption in the periodontium [6]. This activity increases with enhanced glycation levels and poor glycaemic control, thus stimulating further bone resorption and reduced deposition that contributes to the increased levels of PD and alveolar bone loss seen in diabetics [4].
When dental plaque is not removed, after 7 to 10 days gingival inflammation begins [7]. However, this is not a classic infection because no single causative organism has been identified and Koch’s postulates have not been met. This bacterial challenge is a persistent source of inflammatory mediators and leads to endothelial dysfunction [8]. It is the severity of the hyperglycaemia that affects the periodontium most [9]. People living with diabetes have reduced healing capacity. This explains the clinical findings of the increased risk of PD in diabetics. Additionally, there is some evidence that patients with PD are at risk of developing type 2 diabetes [10] and pre-eclampsia [11].
These diseases affect each other with PD having an adverse but modifiable effect on glycaemic control [12]. Periodontal therapy improves metabolic control so the overall management of DM may improve [13,14]. These factors underline the importance of screening diabetics for PD and vice versa.
Periodontal Disease
In summary, PD is the most common disease known. It is an inflammation of both the soft and bony tissues around the teeth. The gingivae become red and swollen, the pocket of soft tissue around the neck of the tooth increases in depth and calculus begins to form below the gum margin. Bone resorbs and spaces appear below the teeth, plaque is retained and in the absence of dental treatment the process can continue until teeth become loose in their sockets and may require extraction. In contrast, normal gingivae are pink, firm and stippled. Bleeding on brushing is never normal.
PD is not a typical bacterial infection and does not meet Koch’s postulates. No single organism has ever been identified as the cause. It is a hypersensitive reaction to toxic products produced by Gram-negative bacteria in mature plaque [6]. This chronic bacterial challenge is a persistent source of inflammatory mediators leading to endothelial dysfunction [15]. It is the severity of the hyperglycaemia that affects the periodontium most [16].
When plaque is not removed for 7-10 days, gingival inflammations follow and this leads to PD [7]. The disease can progress due to these inadequate brushing techniques. This leads to more bone loss and gingival recession over time, adult periodontitis. About 15 percent of the population have a more aggressive type, rapidly progressive periodontitis that may have a genetic element, and as its name suggests, leads to faster breakdown of the periodontium and earlier tooth loss. Inflamed gingivae form sub-gingival calculus. Unless this is professionally removed by ultrasonic scaling, it migrates down the root over time, enhancing bone loss.
Periodontal Risks for Diabetics
PD is the sixth complication of DM and affects its outcome [17]. In summary from the limited studies that have been undertaken:
Cardiac and arterial disease
Poor oral health is associated with atherosclerotic cardiovascular disease raising morbidity fourfold and is associated with chronic infection [18]. Mediators from this chronic infection may lead to endothelial dysfunction [6].
Nephropathy
About 40 per cent of haemodialysis patients are diabetics who are at greater risk of developing PD [19]. For patients with severe PD there is a 2.6 times greater risk of developing kidney problems and end stage renal disease [20]. Periodontal management can contribute to the prevention of severe renal disease [21]. Diabetics could be screened for PD before acceptance onto these programmes [21].
Neuropathy
This is a microvascular complication and associated with xerostomia or dry mouth, that can affect 40 per cent of diabetics [22]. As salivary flow reduces, the risk of developing caries increases due to a reduced buffering capacity and raised salivary sugar levels. There is an inverse relationship between salivary flow and HbA1c levels that may be due to disturbances in glycaemic control [23].
Retinopathy
An increase in the severity of diabetic retinopathy has been associated with the components of PD [24].
Dental care is an important component of diabetes management
for patients. In the UK this was finally recognised in June 2022 by
the National Institute for Clinical Excellence, the Government body
defining care standards for health care professionals. Periodontal
health has now been included in the annual doctor’s checklist for
patients living with diabetes [25] to advise adults with both type 1
and type 2 diabetes at their annual review:
a. That they will be at higher risk of periodontitis
b. That if they get periodontitis, managing it can improve
their blood sugar control and can reduce their risk of hyperglycaemia
c. To have regular oral health reviews at a frequency based
on their oral health needs.
In the longer term this will enable doctors and dentists and
their teams to work together and should provide important benefits
for all those living with diabetes. In the immediate future it raises
questions about:
i. What dental information is relevant for doctors?
ii. How it could be presented in an easily understood way?
iii. What information should doctors provide for dentists?
iv. How could diabetics be involved themselves in sharing
information between doctors, dentists and themselves?
Medical Risks Important for Dentists and Diabetics
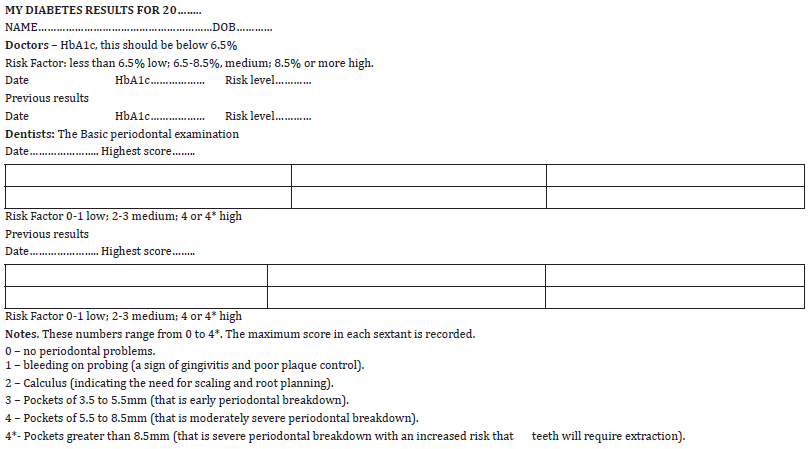
While continuous blood sugar monitoring may be the future, at the present the gold standard for doctors is to maintain the serum HbA1c level below 6.5 per cent. This test gives a three-month assessment of blood sugar levels. Below 6.5 per cent the risks of diabetic complications can be classified as low or green. For levels of 6.5 to 8.5 per cent the risk is moderate or amber, and above 8.5 per cent the risk becomes high or red. Dentists should add these scores to their medical history records and also ask if the diabetic type is 1 or 2.
Dental Risks Important for Doctors and Diabetics
Studies suggest that most doctors have been taught little about PD [25]. From the various indices of periodontal health that have been describe, the measure of choice is the World Health Organisation’s internationally recognised periodontal screening program called the Community Index of Periodontal Treatment Needs (CPITN) that is recorded using a specially designed colour coded pocket measuring probe [26]. This gives six numbers, one in each of three sections comprising molars and premolars on each side and incisors and canines as the central number. This applies to both the maxilla and the mandible making six scores in all. Each section is called a sextant. The maximum score from each sextant is recorded.
The scores, using the original WHO classification for simplicity
are:
i. 0 – no periodontal problems.
ii. 1 – bleeding on probing (a sign of gingivitis and poor
plaque control).
iii. 2 – Calculus (indicating the need for scaling and root
planning by a dentist or hygienist).
iv. 3 – pockets of 3.5 to 5.5mm (that is early periodontal
breakdown).
v. 4 – pockets of 5.5 to 8.5mm (that is moderately severe
periodontal breakdown).
vi. 4* - Pockets greater than 8.5mm (that is severe
periodontal breakdown with an increased risk that teeth will
require extraction).
From these scores the maximum score from one sextant is used. Scores of 0 to 1 indicate low risk, 2 to 3 moderate risk and 4 or 4* high risk. Using these scores will provide an easily understood method of defining the relative risks into low or green, moderate or amber and high or red categories for doctors, dentists and their patients providing that the results are shared and each party knows about them. Two consecutive scores should be recorded to establish trends in disease control. Dentists have been taught to ask their patients if they are diabetic and to record the name of their GP. The reverse is usually not the case. However, doctors and their team will need to do so in future. This should lead to a multidisciplinary approach to care in the longer term.
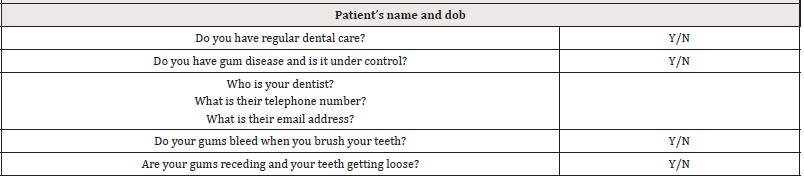
A simple dental questionnaire has been developed for doctors to investigate the dental status of their diabetic patients (Table 1) [27]. This could be expanded to include the CPITN score for those patients receiving dental care. For those patients who do not receive dental care, doctors should consider a suitable referral.
A further question could be: Do you know your CPITN scores?
To date such referrals from doctors to dentists have been uncommon. Doctors should, in the first instance, only identify diabetics who have not had dental care who are in the highest HbA1c risk category for referral for periodontal screening.
This approach should satisfy in part the recent calls that have been made for better co-operation between doctors and dentists in the care of diabetics and the sharing of results [27,28].
Dental Care
People living with diabetes can help themselves to minimise their risks. Fortunately, PD is both a treatable and preventable disease the most important component of which is daily efficient and effective control of plaque by patients. Electric toothbrushes are more likely to remove plaque than hand brushing. Dental floss has been shown to be less effective in controlling plaque than had previously been thought. Where there has been gingival recession the optimum method is the use of interspace brushes making sure that the correct diameter brush is used for each space. However, there is no correlation between the handle colours and sizes for different brush manufacturers.
It is common practice for patients to be prescribed just two sizes of interspace brush, one large and one small, and left to work out for themselves which size to use in each interdental space. To be effective there needs to be slight resistance when the brush is pushed backwards and forwards to mechanically clean each space of plaque. It follows that if a small brush is used in a large space, then this plaque cannot be completely removed, and despite regular professional ultrasonic cleaning to remove biofilm that has a time-limited 7-to-10-day effect [8] before the conditions for Gram negative bacteria in plaque to produce their toxins are reestablished.
The Chooseabrush™ method allows patients to check each space from a range of eight different interspace brush sizes, then record their results on a user-friendly chart to create their own unique record of which brush to use where. In some places, where further resolution of periodontal disease occurs it may prove possible to use a larger size brush. Where calculus has developed then professional ultrasonic scaling to remove these deposits and biofilm from the roots is necessary. Loose teeth may require extraction.
Plaque control also removes the bacteria associated with tooth decay or caries. People living with diabetes may have a raised level of sugar in saliva. If salivary flow and its buffering capacity is reduced because of a dry mouth the risk increases. It also increases when fruit juices are swallowed because these bacteria break down sugar to form acids that begin to decalcify teeth within about a minute and this can last for up to twenty minutes. For those who do have dental care it is important that they attend for review appointments at the time intervals recommended by their dentist as determined by their own disease levels and responses to treatment [29].
Conclusion
Action Plan for Healthcare Professionals
1. Doctors need to recognise that periodontal disease is
the sixth complication of DM and has an important influence on
glycaemic control. They should ask their patients with diabetes
questions about their dental health and dental attendance. They
will have to understand a simple screening measure for periodontal
disease such as CPITN.
2. If their patients do not have dental care and are in the
highest risk category with HbA1c levels of 8.5 per cent or over, they
should be advised that dental care may help them and a referral
made for a basic periodontal assessment.
3. Dentists will need to be taught about HbA1c levels and
how this affects medical risks and amend their medical history
forms to ask whether it is type 1 or type 2 diabetes and what the
latest HBA1c score was. They will need to share the CPITN scores
with their patients as a matter of routine.
4. Doctors and dentists need to work more closely together
in the care of diabetics. This includes sharing test and examination
results and developing channels of communication. In addition,
doctors will have to be taught about the basics of periodontal
disease and how its treatment and prevention can help in glycaemic
control. This educational lead should come from dentists [30] and
could include:
a. Deans of dental schools contacting their medical
counterparts and arranging for student lectures.
b. Individual dentists contacting their diabetic patient’s
doctor with their periodontal results.
c. Learned medical societies arranging postgraduate
lectures.
5. For diabetes patients in the high-risk category who have
been advised by their doctor to seek dental care could lead to audit
to identify if the advice has been taken and what effect dental
treatment has had on glycaemic control.
Action Plan for Diabetics
Diabetics should be encouraged to know about their HbA1c and CPITN scores and share them with their respective professional advisors as a first stage in developing better interprofessional co-operation. Their results form (Table 2) has been designed to simplify the objective of information sharing between the three parties, doctors, dentists and diabetics because the traffic light system is readily understood by all [31]. It should be regarded as a starting point in helping diabetics to be more aware of their risks of complications and make them more involved in their care. The CPITN scores have been purposefully included because, in the short term, doctors will not be aware of their significance, and, if current UK practice is followed in other countries, patients will not be aware of their results.
Conflict of interest
The author is the inventor of the Chooseabrush™ interdental plaque patient-focussed charting methods.
References
- Stöhr J, Barbaresko J, Manuela Neuenschwander, Sabrina Schlesinger (2021) Bidirectional association between periodontal disease and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Scientific Reports 11(1): 13686.
- Battancs E, Gheorghita D, Nyiraty S, Lengyel C, Eördegh G, et al. (2020) Periodontal Disease in Diabetes Mellitus: A Case–Control Study in Smokers and Non-Smokers. Diabetes Therapy 11(11): 2715-2728.
- O’Connor JC, Sherry CL, Guest CB, Freund GG (2007) Type 2 diabetes impairs insulin receptor substrate-2-mediated phosphatidylinositol 3-kinase activity in primary macrophages to induce a state of cytokine resistance to IL-4 in association with overexpression of suppressor of cytokine signaling-3. Journal of Immunology 178(11): 6886–6893.
- Liu R, Bal HS, Desta T, Yugal Behl, Dana T Graves (2006) Tumor necrosis factor-alpha mediates diabetes-enhanced apoptosis of matrix-producing cells and impairs diabetic healing. The American Journal of Pathology 168(3): 757–764.
- Southerland JH, Taylor GW, Offenbacher S (2005) Diabetes and Periodontal Infection: Making the Connection. Clinical Diabetes 23(4): 171-178.
- Cekici a, Kantaria A, Hasturk H, Van Dyke E (2014) Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000 64(1): 57-80.
- Löe H, Thielade E, Jensen SB (1965) Experimental Gingivitis in Man. Periodontol 36(3): 177-187.
- Khumaedi AI, Purnamasari D, Wijaya P, Soeroso Y (2019) The relationship between diabetes, periodontal and cardiovascular disease. Diabetes Metab Syndr 13(2): 1675-1678.
- Genko RJ, Borgnakke WS (2013) Risk Factors for periodontal disease. Periodontol 2000 62(1): 59–94.
- Genco RJ, Graziani F, Hasturk H (2020) Effects of periodontal disease on glycaemic control, complications and incidence of diabetes mellitus. Periodont 2000 83(1): 59-65.
- Kumar A, Sharma DS, Verma M, Arundeep Kaur Lamba, Madhavi M Gupta, et al. (2018) Association between periodontal disease and gestational diabetes mellitus: a prospective cohort study. J Clin Periodontol 45(8): 920-931.
- Taylor GW (2001) Bidirectional inter-relationship between diabetes and periodontal disease: an epidemiological perspective. Ann Periodontol 6(1): 99-112.
- Costa KL, Taboza ZA, Angelino GB, Silveira VR, Montenegro R et al. (2017) Influence of Periodontal Disease on Changes of Glycated Hemoglobin Levels in Patients with Type 2 Diabetes Mellitus: A Retrospective Cohort Study. J Periodontol 88(1): 17-25.
- Wang TF, Jen IAn, Chou C, Lei YP (2014) Effects of Periodontal Therapy on Metabolic Control in Patients with Type 2 Diabetes Mellitus and Periodontal Disease. Medicine 93(28): e292.
- Löe H (1999) Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 16(1): 329-334.
- Gianos E, Jackson EA, Tejpal A, Karen Aspry, James O'Keefe, et al. (2021) Oral health and atherosclerotic cardiovascular disease: a review. Am J Prev Cardiol 7: 100179.
- Mahajan S, Bhaskar N, Kaur RK, Jain A (2021) A Comparison of oral health status in diabetic and non-diabetic patients receiving haemodialysis - a systematic review and meta-analysis. Diabetes Metab Syndr 15(5): 102256.
- Preshaw PM, Alba Al, Herrera D, S Jepsen, A Konstantinidis, et al. (2012) Periodontitis and diabetes: a two-way relationship. Diabetologia 55(1): 21-31.
- Yoshioka M, Okamoto y, Murata M, Makoto Fukui, Shizuko Yanagisawa, et al. (2020) Association between oral health status and diabetic nephrology- related indices in Japanese middle-aged men. J diabetes Res 2020: 4042129.
- Bornakke WS, Anderson PF, Shannon C, Jivanescu A (2015) Is there a relationship between oral health and diabetic neuropathy? Curr Diab Rep 15(11): 93.
- P A Moore, J Guggenheimer, K R Etzel, R J Weyant, T Orchard (2001) Type 1 diabetes, xerostomia and salivary flow rates. Oral Surg Oral Med Oral Path Oral Radiol Endodod 92(3): 281-291.
- Kamath Y, Tandon A, Goash, S, Sarpangala S, Bhandary S (2020) The association between diabetic retinopathy and periodontal disease. Saudi Journal of Ophthalmology 34(3): 167-170.
- (2015) Type 1 diabetes in adults: diagnosis and management | Guidance | NICE.
- (2015) Type 2 Diabetes in adults: Management | Guidance | NICE.
- Tse SY (2018) Diabetes and periodontal awareness and practice among doctors working in public general outpatients clinics in Kowloon West Cluster. Fam Prac 19(1): 199.
- Barmes D (1994) CPITN a WHO initiative. Int Dent J 44(5 Suppl 1): 523-525.
- Turner CH (2021) Diabetes mellitus and dental health: a review. Geriatric Med J
- Siddiqi J, Zafar S, Sharma A, Quaranta A (2020) Diabetes mellitus and periodontal disease: The call for inter-professional education and inter-professional collaborative care. J Interprof Care 36(1): 93-101.
- Turner CH (2022) Interdental brushes and ISO standards Brit dent J 232(11): 761-762.
- (2004) Overview | Dental checks: intervals between oral health reviews | Guidance | NICE.
- Turner CH (2022) Diabetes mellitus and periodontal disease: the professions choices. Brit dent J 233(7): 537-538.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.